space cadet
SENIOR MEMBER
I have been saying this for a while, they need to give all that stuff away, as soon as possible
 www.nbcnews.com
www.nbcnews.com
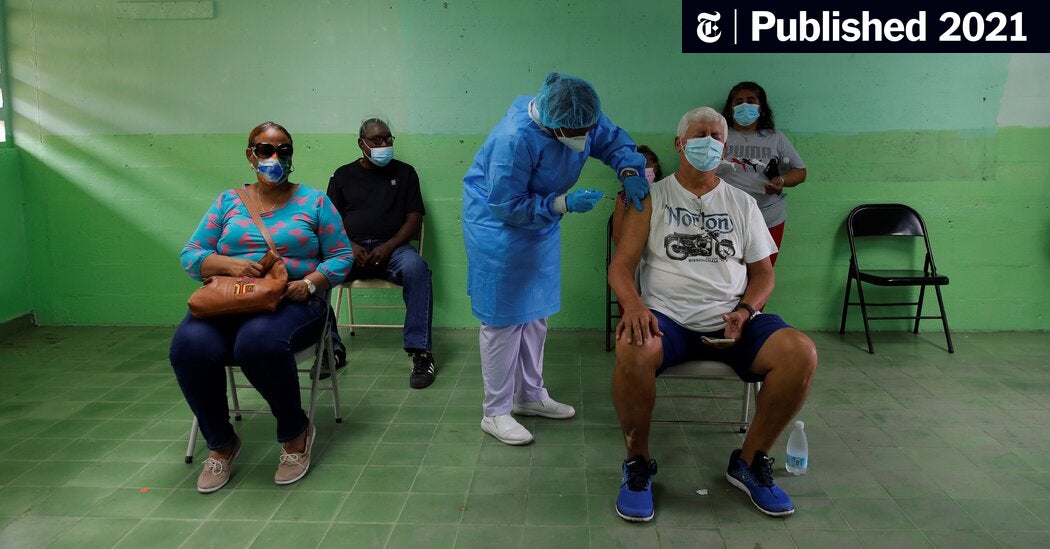
WASHINGTON — The United States plans to ship its stockpile of millions of AstraZeneca vaccine doses overseas, a move aimed at helping other countries struggling with a lack of doses to vaccinate their populations.
White House Covid-19 adviser Andy Slavitt said in a tweet Monday that 60 million doses of the vaccine would be sent to other countries “as soon as they become available.”
The decision comes as the pandemic has spiked in India, where thousands are dying daily as the nation’s stressed hospitals struggle to treat the virus. President Joe Biden spoke to India's Prime Minister Narendra Modi Monday and administration officials said Monday they are sending a range of supplies to India, including oxygen equipment, raw materials used in vaccine production, rapid testing kits, and the treatment Remdesivir.
Public health officials, lawmakers and world leaders have been urging the U.S. to release some of its stockpile of the AstraZeneca vaccine to other countries that have cleared it for use while American reviews of safety and efficacy data continue. Slavitt didn't mention names of countries to which the vaccine doses would be sent.
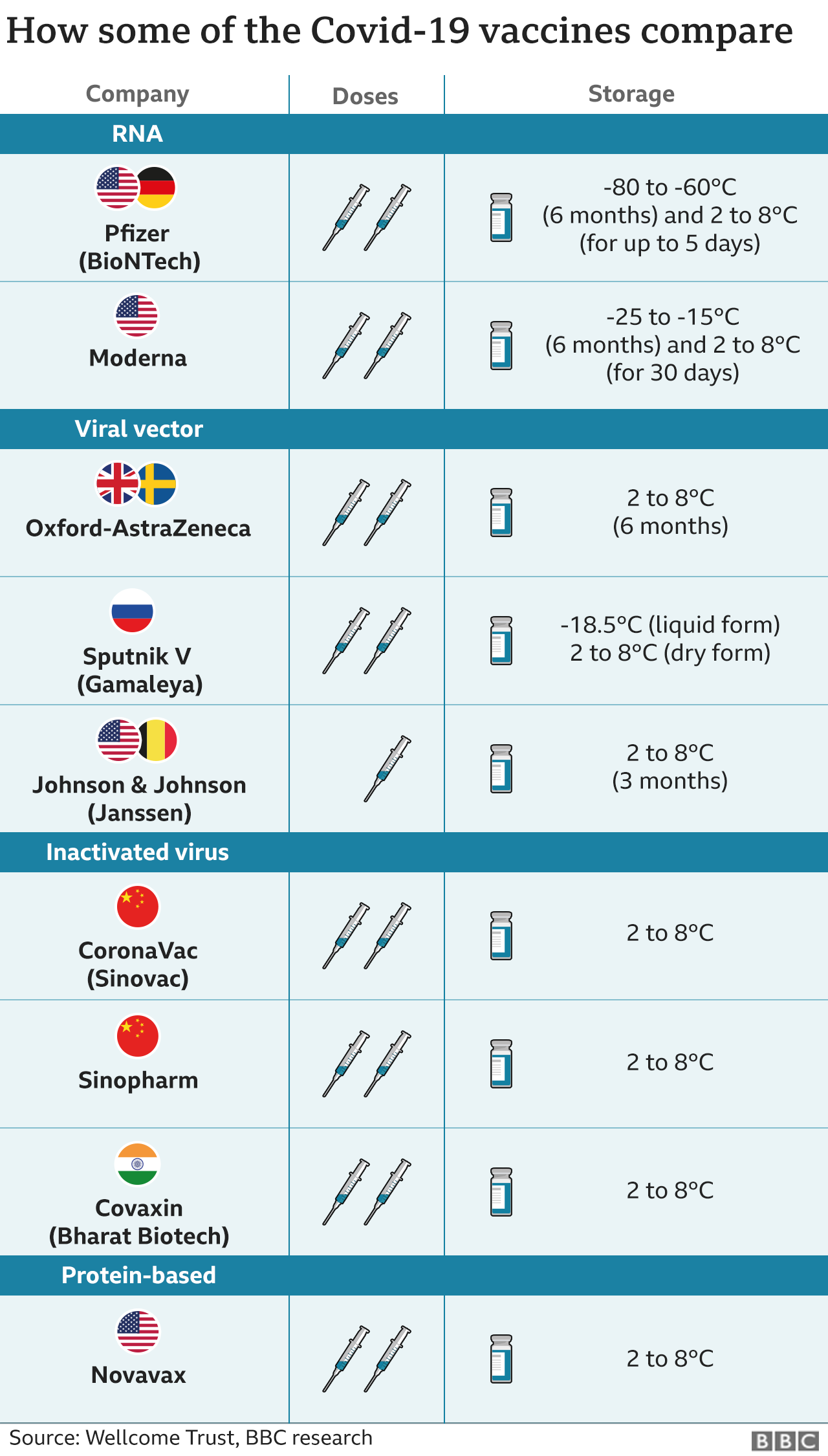
White House press secretary Jen Psaki said no doses will be shared until the Food and Drug Administration has concluded its review, which should take several more weeks. She said the U.S. currently has 10 million doses and expects an additional 50 million to be delivered by the company during May and June.
"Given that AstraZeneca is not authorized for use in the United States, we do not need to use AstraZeneca in our fight against Covid over the next few months," she said. "Before any AstraZeneca doses are shipped from the United States, the FDA will confirm any such doses meet its expectation for product quality.”
The AstraZeneca vaccine has suffered from delays after "very rare" incidents of blood clots and questions over the data the company submitted to the FDA.
The Biden administration had said it would wait to share significant numbers of its doses with other countries until it had ensured enough supply for Americans. But in recent days, administration officials have said they are confident they will have enough doses of the Pfizer and the Modern vaccines to vaccinate every U.S. adult who wants one. The two companies have agreed to supply a total of 600 million doses by the end of July.
An administration official told reporters said they are confident the U.S. will have enough supply from the three other available vaccines to meet the needs of Americans for the next several months.
Use of the Johnson & Johnson vaccine restarted this week after a pause to study a rare side effect of blood clots.
At the same time, the U.S. rate of vaccinations has declined over the past week and administration officials have said supply currently outstrips the demand.
The U.S. has already committed to sharing 4 million doses of the AstraZeneca vaccine with Canada and Mexico.
Rep. Raja Krishnamoorthi, D-Ill., who sits on the House Select Subcommittee on the Coronavirus Crisis, and other lawmakers have been urging the U.S. to share its stockpile of doses with India.
“We cannot allow these vaccine doses to go unused, we should send them abroad where they can be used and they can save lives, right now,” he said on MSNBC.
India’s coronavirus infections have set a new world record in recent days with more than 350,000 new infections within 24 hours. Hospitals have said they are overwhelmed and begging for oxygen supplies as a humanitarian crisis unfolds in the world’s second most populous country.

U.S. to share 60 million doses of AstraZeneca Covid vaccine with other countries
White House Covid adviser Andy Slavitt said in a tweet Monday that doses would be sent to other countries “as soon as they become available.”
U.S. to share 60 million doses of AstraZeneca Covid vaccine with other countries
White House Covid adviser Andy Slavitt said in a tweet Monday that doses would be sent to other countries “as soon as they become available.”WASHINGTON — The United States plans to ship its stockpile of millions of AstraZeneca vaccine doses overseas, a move aimed at helping other countries struggling with a lack of doses to vaccinate their populations.
White House Covid-19 adviser Andy Slavitt said in a tweet Monday that 60 million doses of the vaccine would be sent to other countries “as soon as they become available.”
The decision comes as the pandemic has spiked in India, where thousands are dying daily as the nation’s stressed hospitals struggle to treat the virus. President Joe Biden spoke to India's Prime Minister Narendra Modi Monday and administration officials said Monday they are sending a range of supplies to India, including oxygen equipment, raw materials used in vaccine production, rapid testing kits, and the treatment Remdesivir.
Public health officials, lawmakers and world leaders have been urging the U.S. to release some of its stockpile of the AstraZeneca vaccine to other countries that have cleared it for use while American reviews of safety and efficacy data continue. Slavitt didn't mention names of countries to which the vaccine doses would be sent.
White House press secretary Jen Psaki said no doses will be shared until the Food and Drug Administration has concluded its review, which should take several more weeks. She said the U.S. currently has 10 million doses and expects an additional 50 million to be delivered by the company during May and June.
"Given that AstraZeneca is not authorized for use in the United States, we do not need to use AstraZeneca in our fight against Covid over the next few months," she said. "Before any AstraZeneca doses are shipped from the United States, the FDA will confirm any such doses meet its expectation for product quality.”
The AstraZeneca vaccine has suffered from delays after "very rare" incidents of blood clots and questions over the data the company submitted to the FDA.
The Biden administration had said it would wait to share significant numbers of its doses with other countries until it had ensured enough supply for Americans. But in recent days, administration officials have said they are confident they will have enough doses of the Pfizer and the Modern vaccines to vaccinate every U.S. adult who wants one. The two companies have agreed to supply a total of 600 million doses by the end of July.
An administration official told reporters said they are confident the U.S. will have enough supply from the three other available vaccines to meet the needs of Americans for the next several months.
Use of the Johnson & Johnson vaccine restarted this week after a pause to study a rare side effect of blood clots.
At the same time, the U.S. rate of vaccinations has declined over the past week and administration officials have said supply currently outstrips the demand.
The U.S. has already committed to sharing 4 million doses of the AstraZeneca vaccine with Canada and Mexico.
Rep. Raja Krishnamoorthi, D-Ill., who sits on the House Select Subcommittee on the Coronavirus Crisis, and other lawmakers have been urging the U.S. to share its stockpile of doses with India.
“We cannot allow these vaccine doses to go unused, we should send them abroad where they can be used and they can save lives, right now,” he said on MSNBC.
India’s coronavirus infections have set a new world record in recent days with more than 350,000 new infections within 24 hours. Hospitals have said they are overwhelmed and begging for oxygen supplies as a humanitarian crisis unfolds in the world’s second most populous country.